In Paris , 196 countries agreed to develop plans to reduce their carbon emissions in such a way as to keep global warming below 1.5°C. Although each country will develop its own plan, the best plan for the US, and many other countries, would be a carbon fee and dividend system such as that developed by the Citizens’ Climate Lobby (CCL), which has broad bipartisan support. CCL’s proposal would place a fee on carbon at the source, and market forces would then encourage reduced emissions, energy conservation and investments in renewable energy. The fee collected is not a tax as it would be distributed equally to every household as a monthly energy dividend.
CO2 emissions: CCL’s legislative proposal would set an initial fee on carbon at $15 per ton of CO2 emission or CO2 equivalent emissions with the fee increasing by $10 each year until the US emissions drop to 1990 levels. The main contributors to CO2 are combustion of coal, natural gas, and gasoline, with minor equivalent emissions coming from other industrial chemicals. A little chemistry allows us to calculate the tons of CO2 that a ton of each fuel produces.
Coal: It is hard to calculate coal’s contribution exactly as it has from 65% to 95% carbon and the rest is impurities. Those include mercury, cadmium, lead, manganese, selenium, sulfur, nitrogen, and some radioactive elements. Much of the environmental damage and many cases of lung disease can be traced to the impurities and to the mining of coal. For calculation purposes we will assume that coal is all carbon as graphite, but keep in mind that each source of coal is different.
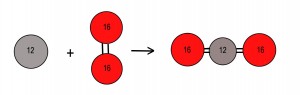
The chemically equation for the reaction of carbon with oxygen is:
 http://jcmooreonline.com/wp-content/uploads/2016/01/co22--768x244.jpg 768w, http://jcmooreonline.com/wp-content/uploads/2016/01/co22--1024x325.jpg 1024w, http://jcmooreonline.com/wp-content/uploads/2016/01/co22-.jpg 1497w" alt="co22" width="400" height="127">
http://jcmooreonline.com/wp-content/uploads/2016/01/co22--768x244.jpg 768w, http://jcmooreonline.com/wp-content/uploads/2016/01/co22--1024x325.jpg 1024w, http://jcmooreonline.com/wp-content/uploads/2016/01/co22-.jpg 1497w" alt="co22" width="400" height="127">
Carbon + Oxygen => Carbon Dioxide
The mass of each atom or molecule in atomic mass units (MU) is written on the atom. The equation says that 12 mass units of carbon react with 32 mass units of oxygen to produce 44 mass units of carbon dioxide. The equation is like a recipe and once you establish the basic relationship, it can be scaled up to tons quite easily, i.e. :
C + O2 => CO2
12 MU Carbon + 32 MU Oxygen => 44 MU Carbon Dioxide – or –
12 Tons Carbon + 32 Tons Oxygen => 44 Tons Carbon Dioxide
Thus, each ton of carbon produces 3.6 tons of carbon dioxide.
Natural gas: Natural gas is composed mostly of methane, CH 4 , with small impurities of other hydrocarbon gases. Following the method above:
 http://jcmooreonline.com/wp-content/uploads/2016/01/Rx-768x203.jpg 768w, http://jcmooreonline.com/wp-content/uploads/2016/01/Rx-1024x271.jpg 1024w, http://jcmooreonline.com/wp-content/uploads/2016/01/Rx.jpg 1535w" alt="Rx" width="550" height="145">
http://jcmooreonline.com/wp-content/uploads/2016/01/Rx-768x203.jpg 768w, http://jcmooreonline.com/wp-content/uploads/2016/01/Rx-1024x271.jpg 1024w, http://jcmooreonline.com/wp-content/uploads/2016/01/Rx.jpg 1535w" alt="Rx" width="550" height="145">
CH 4 + 2O 2 => CO2 + 2H2O
16 MU Methane + 64 MU Oxygen => 44 MU Carbon Dioxide +36 MU of Water
16 Tons Methane + 64 Tons Oxygen => 44 tons Carbon Dioxide +36 tons of Water
Each ton of methane produces 2.8 tons of carbon dioxide.
Gasoline: Gasoline is composed of many volatile liquid compounds, but it can best be represented as octane, which has eight carbon atoms and 18 hydrogen atoms, C 8 H 18 . (The model for Octane is large so here we will just work from the equation. )
C 8 H 18 + 25/2 O 2 => 8CO2 + 9 H2O
114 AMU Octane + Oxygen => 352 AMU Carbon Dioxide + Water
114 Tons Octane + Oxygen => 44 tons Carbon Dioxide + Water
Each ton of octane produces 3.1 tons of carbon dioxide.
Note: One gallon of gasoline is about 7 pounds and it produces about 21 pounds of CO2. That means that 95 gallons of gasoline will produce 1 ton of carbon dioxide. The $15 per ton carbon fee would increase the cost of 95 gallons of gas from about $200 to about $215, or about 7%.
Heat of Combustion: Each fuel releases a different amount of energy when burned, measured in kilojoules of energy per mole of fuel burned. Those are listed below along with another important quantity, the amount of heat released per mole of carbon dioxide released.
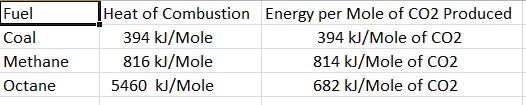 http://jcmooreonline.com/wp-content/uploads/2016/01/Fuel.jpg 526w" alt="Fuel" width="526" height="105">
http://jcmooreonline.com/wp-content/uploads/2016/01/Fuel.jpg 526w" alt="Fuel" width="526" height="105">
Note that Methane releases more than twice as much energy as coal for each mole of carbon dioxide produced. This was the impetus to convert coal-fired power plants to natural gas-fired plants. That would help in the short term as natural gas has fewer impurities and produces more energy per mole of CO2 released. However, there is another factor to be considered which is the Global Warming Potential of each greenhouse gas.
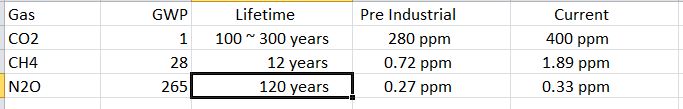
Global Warming Potential (GWP ): The amount that each greenhouse gas contributes to global warming depends upon its concentration in the atmosphere, it’s effectiveness at trapping heat, and its lifetime in the atmosphere. The focus is on carbon dioxide as it is the greenhouse gas whose concentration has increased the most by burning fossil fuels. Methane is very efficient at trapping heat and has a GWP 28 times that of CO2. Though methane’s concentration is low, it has more than doubled since pre-industrial times. There are other greenhouse gases which are more effective at trapping heat and have longer lifetimes, such as N2O, but their contributions are small because they have low or stable concentrations. Below is a table comparing those. Source.
 http://jcmooreonline.com/wp-content/uploads/2016/01/co2-table.jpg 683w" alt="co2 table" width="683" height="109">
http://jcmooreonline.com/wp-content/uploads/2016/01/co2-table.jpg 683w" alt="co2 table" width="683" height="109">
Although converting coal-fired power plants to natural gas might be advantageous in the short term, we should be concerned about methane’s volatile prices, the link between fracking and earthquakes, and its GWP. Large amounts of methane are lost from fracking operations, leaking gas wells, and pipeline leaks. If even 4% of the methane produced is lost to leaks, then any advantage of converting to methane will be lost. The EPA has taken steps to reduce methane loss to the air, but is a very difficult thing to measure . One study found that infrastructure leaks in the Boston area accounted for about 2.6% of the methane transmitted. And methane, when burned, still ends up as CO2 in the atmosphere. You can see from the table that the amount of methane in the air is growing, and rather than count on it for the future, we should focus on converting to renewable energy sources as quickly as possible.
(C) 2016 – J.C. Moore
Note: Here is a model of octane for the curious:
 http://jcmooreonline.com/wp-content/uploads/2016/01/octane.jpg 322w" alt="octane" width="322" height="137">
http://jcmooreonline.com/wp-content/uploads/2016/01/octane.jpg 322w" alt="octane" width="322" height="137">






