Does burning coal have to be a bad thing ?

LINK :
http://nextbigfuture.com/2014/11/ending-age-of-steam-with-supercritical.html
November 04, 2014
Ending the age of steam with Supercritical CO2
The age of steam has generally referred to the use of the Steam engine from 1770 to 1914 However, for power generation, we have not left the age of steam. It will take the next several decades to scale up supercritical CO2 turbines. The other class of technologies for ending the age of steam would be to directly convert fast-moving charged particles [from fusion power] directly into electrical current.Wind, solar and hydro also do not involve steam but they have not eliminated steam turbine power from fossil fuels.
Toshiba Corporation announced that it will supply a first-of-a-kind supercritical CO2 turbine to a demonstration plant being built in Texas, USA. The plant will be developed by NET Power, LLC, a U.S. venture, together with CB and I, the most complete energy infrastructure focused company in the world, Exelon Corporation, one of the leading competitive energy providers in the U.S., and 8 Rivers Capital, the inventor of the unique supercritical CO2 power cycle that will be demonstrated by this plant. The turbine is an essential part of the system, and Toshiba will start delivering the key equipment in August 2016. The plant is expected to enter the commissioning stage later in 2016.
Supercritical CO2 could make coal plants up to 40% more efficient and enable capture of pipeline ready CO2.
Supercritical CO2 technology can make solar and nuclear power more efficient and lower cost as well. Those energy sources would not require the efficiency gains to be used on CO2 capture and storage.
The US National Energy Technology Lab lists the Dept of Energy funded Supercritical CO2 projects.
Instead of 40% cheaper electricity using efficiency to pay for cost of Capturing CO2
New Supercritical CO2 power cycle could utilize pressurized oxy-combustion in conjunction with cryogenic compression to achieve the DOE goals of 90 percent CO2 removal at no more than a 35 percent increase in cost of electricity (COE) as well as high overall plant efficiencies with CO2 compression to 2,200 psia. Supercritical CO2 would use the 40% efficiency gain of going to about 800 degrees celsius to enable CO2 90% removal at the same cost as current coal or natural gas plants.
The technological challenge and efficiency gains of leaving steam technology is proposed to be spent on capturing CO2.
Nextbigfuture reviewed the supercritical CO2 turbine roadmap. The Toshiba work is executing to the dates on that roadmap.
* Sandia National Laboratories and Lawrence Berkeley National Laboratory are involved with Toshiba, Echogen, Dresser Rand, GE, Barber-Nichols in S-CO2 cycles.
* Toshiba, The Shaw Group and Exelon Corporation are engaged in a consortium agreement to develop Net Powers gas-fired generation technology with zero emissions target. This approach uses an oxy-combustion, high pressure, S-CO2 cycle, named Allam Cycle. Toshiba will design, test and manufacture a combustor and turbine for a 25MW natural gas-fired plant. A 250MW full-scale plant is expected by 2017.
* Echogen Power Systems has been developing a power generation cycle for waste heat recovery, CHP, geothermal and hybrid as alternative to the internal combustion engine.
* Pratt and Whitney Rocketdyne is engaged with Argonne National Laboratories in a project with aim to integrate a 1000 MW nuclear plant with a S-CO2 cycle.
The reasons of growing interest toward this technology are manifold:
* simple cycle efficiency potentially above 50%;
* near zero - emissions cycle;
* footprints one hundredth of traditional turbomachinery for the same power output due to the high density of working fluid;
* extraction of pipeline ready CO2 for sequestration or enhanced oil recovery, without both CO2 capture facilities and compression systems;
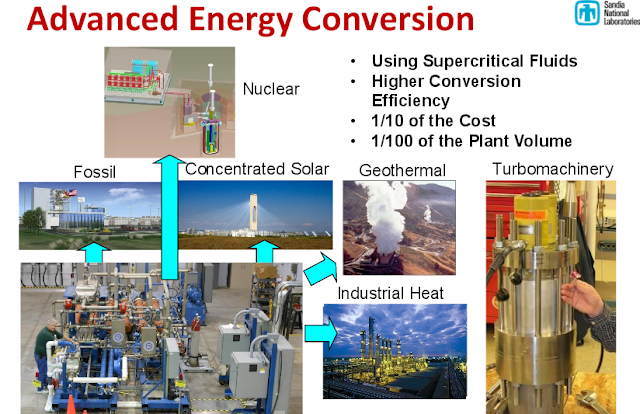
* integration with concentrating solar power (CSP), waste heat, nuclear and geothermal, with high efficiency in energy conversion;
* applications with severe volume constraints such as ship propulsion
There is a DOE project to a make a 10 MWe supercritical CO2 turbine that should be completed in 2015.





This high tech approachappears to solve the problems coal is currently perceived to have . Don't let ideology blind your perceptions ...
What do they do with the heavy metals?
Above & beyond that fact is the political desire [especially by Obama ] to completely eliminate coal as a fuel . That is based on ideology not technology . That seems to be a recurring theme from Obama . He wanted to promote solar & wind tech . But there has been little expansion in those as a percentage of total energy usage ... And hardly any expansion in jobs there .
He wanted to expand the use of ethanol as fuel even though a larger %age of it in motor fuel is damaging to gasoline engine systems . He wanted to avoid drilling for more oil but again , technology stepped in and went over his head .
The concept expressed in the seeded article is solidly based on thermodynamic principles : The efficiency of a heat engine depends of the temperature difference between the heated fuel in the turbine and the exhaust . By using CO2 as a compressible fluid makes it straightforward to apply this design concept .
It should come as no surprise that a Japanese company is interested in developing this concept . The advantage of less land use [much less] is obvious in a small island nation like Japan . Have I left out anything ?
I don't have an answer to that but since this process is more efficient it means less heavy metals will be produced per KWH . I expect it would require more technical details to give a proper answer .
Upon further examination it appears that the heavy metals might be removed from the exhaust gases in one stage of the process . If you look at diagram #2 above [single loop CPOC cycle] at the bottom left is a process called "water separation & flue gas clean-up" . It seems likely that removal of such contaminants would be performed at that stage ...
The University of North Dakota EERC has been a leader in clean energy research for many years and have lottsa interesting stuff going on over there. Right now, one of the coldest cities in America, Grand Forks, has much of their heat generated from clean coal technology, including the University, Altru hospital, and many others!
:~)
That does seem likely ... although the biggest problems in their nuclear industry won't be solved by this technology .
Based on the much smaller land footprint required for such plants the Japanese may simply start importing coal . If this technology is as clean as the article implies there would be few downsides to such an action ... although other fuels could be employed as well including LNG , bunker oil , etc .
That's interesting considering how bad the Russian economy is right now . Is that a recent development and can you supply a link ?
I went to the site you linked ... looks interesting . I found this video there which is about CO2 injection into oil wells :
Were there some articles specifically about clean coal ?
There are 2 points I will inquire about :
Can I assume these friends are not at Fukushima ?
How long ago did they make these comments about Russia "messing with" Japan ?
This looks very promising. I am quite impressed. Question, what is the ph of the residual air?
I just saw that myself. The only thing is, that process of cleaning up the water takes (assumption) energy. This is the problem with the scrubbers that are in use currently: They leave a sludge behind that takes a whole bunch of processing to get rid of. I wonder if the energy efficiency calculations have taken this into account?
I also wonder if they did not stumble upon this as an outgrowth of petroleum product extraction and have rebranded it as clean coal technology....
That's nice ... What about it did you find so impressive ? Also do you have any reasons for suspecting the exhaust pH ?
Petey, no, burning coal doesn't have to be a bad thing. This looks like good, and very interesting technology!
However, in KY, mining it can be a bad thing:
This new technology looks as if it would really help with the burning of coal, and I'm all for it! But it does nothing for my state's problems with the mining of coal. Nor does it do anything to get these people out of the coal mine's inertia... The people who work in the mines get paid an annual salary that's pretty good, for unskilled labor. However, they only work 3-6 months a year. They need job training and new kinds of jobs... Not that it will ever happen.
Has anyone ever been to West Virginia?
Everything, and I mean everything, is rusted. The cars, the mailboxes, the guard rails, the gutters, the gutter nails, anything metal that is outside is rusted. Thanks to acid rain...
We have higher emissions standards here in KY, (next door), that reduces the acid rain, so we're not rusty. But acid mine drainage gets us.
Thanks for your experienced perspective Dowser . The article did not talk about sulfur as far as I can tell . Perhaps it is a problem with only some coal ?
Flame ,
Thorium reactors are a completely different technology but one which would be adaptable to this super critical CO2 type of turbine system . However , it would require a major commitment to developing thorium reactor technology . So far there is little to no political will to get involved in such a large project .
Indeed . From my POV it is due to an anti-CO2 emissions ideology . Or maybe an attempt at political retribution against red states . Do you have another perspective on this ?
Well, almoat all coal has some sulfur, but the anthracitic coals are usually lower in sulfur than others. We've mined all of ours. There is some high sulfur coal left, but we can't use it in our plants, because of the emission guidelines-- and none of us want to be rusty. We actually do mine some of the higher sulfur coals, and sell it to WV.
Kentucky's geological survey has just completed a huge study on CO2 sequestration-- where the CO2 is pumped deep underground. It was a huge success, and may offer some real hope for the problem.
I don't see KY spending the money to retool the coal fired plants, in all honesty. We just don't have it. Not to mention, rate increases will be a real problem for those of us, (a LOT of us), who live "on the edge".
mountain top removal creates a LOT of problems!! Here is a before and after picture.
Petey,
It's highly efficient, it handles CO2 emissions well, and we need fuel. My ph question was about acid rain. Right now China can't grow anything in certain areas due to acid rain.
It comes from burning coal. So I was wondering if they have figured a way to make the remaining emissions acid free?
Sorry Petey, I'm so used to the forest, I forgot to point out a few trees!
Check this one out, it gives a little insight into some of their newest and most promising research and implementation in the real world... ...
The process of turning coal into a directly usable liquid biomass is quite promising!
It seems unlikely . The concept requires a large amount of CO2 to accomplish . The best [worst ?] fuel for supplying a lot of CO2 is coal . Are you perhaps hunting for the standard ideological scapegoat ... the oil industry ? It sure sounds that way .
I don't have that info . The article only refers to flue GAS cleanup .
Perrie ,
Good point about the acid rain . Dowser went into a lot more details here :
Apparently it depends on what type of coal is combusted ... whether it is low sulfur or not .
BTW anyone who appreciates efficiency can't be all bad ! [ ; ~ ))
That sounds pretty involved ... so many processes . Here is something else on this topic :
....
While the industry has often used nitrogen and carbon dioxide (CO2) in so-called energized or foamed frack fluids to reduce water usage, recent research has focused on the use of C02 to completely eliminate water used in fracking.
....
The CO2 would be in a chilled form known as a super-critical fluid that is neither solid nor liquid. A major challenge is determining the right viscosity for the CO2, in order that it can do the job that water does now
Since CO2 is used in the extraction of petroleum products, the petroleum industry would, it seams, be a large user of that CO2 emanating from the process. Please note the rather large arrow pointing to "Sequestration Ready CO2" in the upper, right hand side of that same diagram. (For convenience I have put it below, also.) seems like some chemical engineer in killed two birds with one stone, so to speak.
I expect that is accurate . But that doesn't force a conclusion it was THE motivation . In any case there may be more than one industry behind this idea . The inventor has come up with a use for CO2 which was considered just a liability . Are you trrying to find fault because the petroleum industry might be part of the motivation ?
Exothermic and endothermic cycling power generation
Seems there is a patent application out there for this type of technology. I really think we need to look at all methods.
Also seems that super critical CO2 can be used to extract hemp oil. I think I might like this technology.
Seriously though, I remember in high school (40+ years ago) discussing using graphite based reactors as opposed to water based. What would really help is an idea based society rather than an ideology based society.
From Wikipedia:
Supercritical carbon dioxide is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure .
So, in order to achieve this state, it must be under high pressure and temperature.
From your link :
Very interesting ... Thanks for the info Steve .
Thanks for your perspectives Flame & Dowser ...
Seems that what has always been needed is a substance that can absorb a great deal of energy before reaching a critical stage of expansion (explosion). This gives the sluggards more time to wake up and decide something needs to be done.
I am not finding fault with anything. If it produces more energy and provides more efficiency, have at it. It just looks from the diagram like two separate processes that were combined.
This is a new reveal to me . Those phase diagrams are often quite involved . This one in comparatively simple . Pressure > 100 bar and temp > 300 deg K .
I wonder what the transfer rate is for supercritical CO2 . And how much larger is it than water , for example ...
Brolly ,
After looking things over again I admit there may be something to the oil industry theory . The mere fact that the bottom right block shows CH4 as the input could be interpreted that way ...
Looking again, I see that high temp is relative. It looks from the graph that 300 deg K is the low temp boundary. That is 27 deg C or about 80 deg F. Atmospheric temp.
According to the Ideal Gas Laws, when you compress gas, you raise temperature (T). So, to have an isothermal (same temperature) compression of the gas means that the gas must be chilled from outside. That means an expenditure of Energy (E). We Will call this energy expenditure E1.
Next there is a refrigeration heat exchanger, presumably there is some energy used there, because the temp is reduced from 100 deg F to 0 deg F. The energy used in this we will call E2.
Then comes the CO2 cryo-pump, which must just take gads of energy, because it is raising the pressure to 2200 psai @ 20 deg F. This is E3. (By the way, According to my reading, which may be wrong, of the lowest possible temps of the supercritical fluid are above 80 deg F.)
This is, I would believe, the standard method for reacting supercritical SiO2.
Nope , you are good . I get the same result , 80.6 deg F .
I have no doubt there is energy required to perform those operations but supposedly the greater efficiency of the output makes up for it [+ some extra perhaps ] .
Should Have said CO2, not SiO2. And I fell asleep before fully exploring the idea... I was going to ask how, if the supercritical fluid that comes out of the cryo-pump is supposed to be at 20 deg F, just what are they adding to the mix to get it down that far below the supercritical point? Conversely, the graph may just be an approximation....
No worries ... I guessed that the SiO2 was a typo .
"how, if the supercritical fluid that comes out of the cryo-pump is supposed to be at 20 deg F, just what are they adding to the mix to get it down that far below the supercritical point?"
It's possible the material might not be supercritical at that point and only gets there after the next step where it gets heated . Or as you suggested the phase diagram from wiki might be off somewhat . Perhaps the high pressure compensates for the lower temperature?
Things may have changed since then vis a vis Russia & Japan . Russia's economy is going into a steep dive now along with its currency ...
Here are the kinds of problems this concept needs to overcome :
...
....
What happened in Marysville will likely continue in years to come. In the face of an aging fleet of plants, new EPA regulations for air toxics and carbon, and decreasing natural gas costs, coal plants may well become a thing of the past.
....
"There are two primary factors" driving coal plant retirement, says Marco Bruzzano, vice president for strategy and corporate development at DTE Energy. "First is the age of the plants. About a third of our fleet is 50 years old or more, and in the next 10 years we'll have about three-quarters of our fleet more than 50 years old. At some point the level of investment that's required to maintain the plants just makes them uneconomic. Then add on top of that the cost of compliance with new environmental regulations."
The issues of upgrading at low cost and the solution to the potential environmental issues are both dealt with by this technology .
More in the science community are starting to recognize this concept :
LINK :
Can Carbon Dioxide Replace Steam to Generate Power?
Engineers are looking into replacing steam with supercritical carbon dioxide, a technique that could unlock up to 50 percent greater thermal efficiency using a smaller, cheaper turbine.
Credit: Michael Spiller/Flickr
Much has changed in the modern electric power plant since Thomas Edison's era, but the parts that actually turn heat into electrons haven't changed since his eureka moments.
Whether burning coal, concentrating sunlight or splitting atoms, most thermal power plants use the energy for the same thing: heating water into steam to drive a turbine. Steam-based generation produces 80 percent of the world's electricity.
After more than a century of incremental improvements in the steam cycle, engineers have plucked most of the low-hanging fruit and are chasing diminishing returns, spending millions of dollars for every percentage point of efficiency improvement. These upgrades propagate to other steps in electricity production, allowing power plants to extract more work for a given unit of fuel.
In a fossil fuel-fired generator, this means less carbon dioxide emissions for the same unit of electricity produced. For a solar thermal plant, this results in higher capacity at lower operating costs.
Now engineers are looking into replacing steam with supercritical carbon dioxide, a technique that could unlock up to 50 percent greater thermal efficiency using a smaller, cheaper turbine.
Last month, in a budget briefing and in two different hearings before Congress, Energy Secretary Ernest Moniz specifically mentioned the Department of Energy's supercritical carbon dioxide initiatives. The department's 2016 budget request allocates $44 million for research and development on this front, including a 10-megawatt supercritical turbine demonstration system.
A simpler, smaller, cleaner machine
The term "supercritical" describes the state of carbon dioxide above its critical temperature and pressure, 31 degrees Celsius and 73 atmospheres. Under these conditions, carbon dioxide has a density similar to its liquid state and fills containers the way it would as a gas.
Coffee producers are already using supercritical carbon dioxide to extract caffeine from beans. Materials companies are also using it to make plastics and ceramics.
"From a thermodynamic perspective, it's a very good process fluid," said Klaus Brun, machinery director at the Southwest Research Institute, a nonprofit research and development group. "You get a fairly efficient cycle and a reasonable firing temperature."
In its supercritical state, carbon dioxide is nearly twice as dense as steam, resulting in a very high power density. Supercritical carbon dioxide is easier to compress than steam and allows a generator to extract power from a turbine at higher temperatures.
The net result is a simpler turbine that can be 10 times smaller than its steam equivalent. A steam turbine usually has between 10 and 15 rotor stages. A supercritical turbine equivalent would have four.
"We're looking at a turbine rotor shaft with four stages on it that's 4 inches in diameter, 4 feet long and could power 1,000 homes," said Richard Dennis, turbine technology manager at the National Energy Technology Laboratory.
He noted that the idea of a supercritical carbon dioxide power cycle dates back to the 1940s, but steam cycles were already very efficient, well-understood and cheap, creating an uphill slog for a new power block to catch on. In addition, engineers were still finding ways to improve the combustion side of power production, so the need to improve the generation side of the plant wasn't as acute until recently.
Regulations could create an expanding market
Now regulations and climate concerns are forcing power producers to consider ideas like supercritical carbon dioxide.
"In just the closed, indirect cycle, numbers suggest that the thermodynamic efficiency, at similar operating temperatures to a steam cycle, you would see a 3- to 4-percentage-point improvement," Dennis said. With further optimization and better materials, engineers could push performance even higher.
The indirectly heated power block would essentially be a drop-in replacement for a steam power block. Such a device would be a boon for nuclear power stations, concentrating solar farms, geothermal installations, combined heat and power systems, and fossil fuel-fired power plants.
Supercritical turbines would also be an attractive upgrade from steam systems aboard ships and submarines, producing the same power while occupying less space. Because they use carbon dioxide instead of water as their process fluid, these turbines would also work well in drought-stricken areas.
In addition, a supercritical turbine could fit into a directly heated cycle, where a fuel like natural gas burns in the presence of pure oxygen inside the turbine, creating only water and carbon dioxide as waste.
Operators could then remove water and sequester the excess carbon dioxide. "With modest success in the technology management program, these cycles could compete with combined-cycle natural gas turbines and carbon capture and storage," Dennis said. The system could also route waste heat back into the front end of the system, further increasing overall efficiency.
However, the turbine is not the only component of the power block; a supercritical carbon dioxide system still needs heat exchangers, cooling systems and piping, which add to cost and complexity. And carbon dioxide can corrode materials, so engineers will have to redesign much of the plumbing and support hardware from steam systems to account for these problems.
Another concern is that most of the supercritical carbon dioxide systems demonstrated to date were built at kilowatt scales, too small to offer useful lessons for a full-sized version. DOE's pilot proposal would go a long way toward demonstrating the feasibility of supercritical carbon dioxide. "Building a 10-megawatt plant would be a huge, huge step forward," Dennis said.
Reprinted from Climatewire with permission from Environment & Energy Publishing, LLC. www.eenews.net , 202-628-6500
My pleasure . I think the main points are summarized in the comment directly above yours . CO2 reaches a supercritical state at about 88 degrees F and above 73 atmosphere of pressure . Those are not extreme conditions but they provide many large efficiency benefits .
The big thing is that CO2 becomes not the dangerous substance that climatologists rail at but a useful substance worth capturing from the smokestack of power plants .
And here is the location of your seed :
After some inspiration from xxjefferson51 : [seed here ] I decided to bring this article back to the FP . Its been 2 years since I seeded this . And the latest news is that the device has been manufactured & shipped :
November 7, 2016
Toshiba Ships Turbine for Net Power Supercritical CO2 Power Plant