Israeli tech firm develops life-saving automatic kidney monitoring device
Category: Health, Science & Technology
Via: buzz-of-the-orient • 5 years ago • 2 commentsBy: ILANIT CHERNICK

Still ANOTHER amazing medical development from Israel to benefit the rest of the world.

Israeli tech firm develops life-saving automatic kidney monitoring device
Acute Kidney Injury affects 19 million patients worldwide, and result in 300,000 deaths a year in the US.
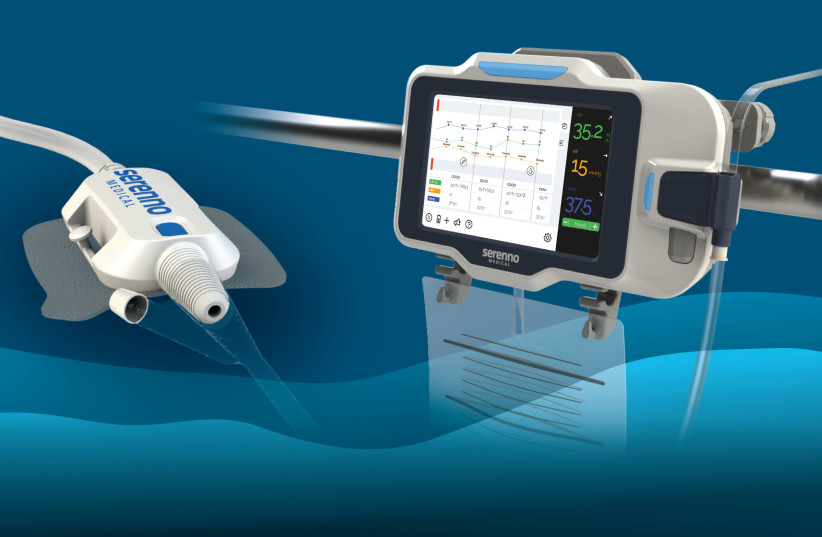
Sentinel urine output monitor for early detection and prevention of Acute Kidney Injury (photo credit: SERENNO MEDICAL
Yokneam based med-tech company Serenno Medical has pioneered a novel and robust device that automatically monitors kidney function and detects kidney damage in hospitalized patients.
According to the Serenno Medical, continuous kidney function assessment allows for the early detection of Acute Kidney Injury (AKI), “a common condition in hospitalized patients that significantly increases risk of mortality during and after hospitalization.”
AKI affects approximately 19 million patients worldwide every year, with 300,000 deaths yearly in the US alone. It occurs in 60% of all ICU patients, and 10% of all hospital admissions.
The company explained that accurate measurement of urine output (UO) is clinically accepted as the best method for monitoring kidney function.
But, the problem is, explained the company’s CEO Tomer Lark, “UO is currently monitored intermittently and manually by ICU nurses.”
“The overworked nursing staff measures a single patient once every hour, sometimes only once every two hours. It is the only parameter still measured manually in the ICU, while the monitoring of all the other vital signs is fully automatic,” Lark told The Jerusalem Post on Wednesday.
This means that events of reduced urine flow indicating possible kidney damage, can go undetected.
“The completely automated Serenno system offers a solution to this problem,” he said. “Using our device, nurses receive dramatically more UO data and are also automatically alerted to any change in kidney function, thus have more time to attend to their patients' needs.”
Asked what inspired the idea, Lark said that it all began when Dr. Manu Malbrain, the ICU director of University Hospital Brussels, approached Serenno's founder Noam Hadas with a problem about how to monitor a different parameter in the ICU called Intra-Abdominal Pressure (IAP).
“During the research phase and while developing the solution to the IAP problem, we discovered a new way to monitor both IAP and UO,” Lark continued. “Monitoring both conditions is critical to the patient and lifesaving. As UO is better understood by most physicians and monitored in all ICU patients, we decided to focus first on this parameter.
“But our device accurately measures both parameters in a fully automatic and continuous manner,” he added.
Lark explained that the kidneys are the body's waste filtering system.
“They clear the body of unwanted materials by separating blood from waste, which is then excreted from the body in the form of urine in a stable and ongoing process,” he stressed. “Changes of little to no urine, even in short durations, are indicative of developing kidney problems.”
But, Lark pointed out, the Serenno system offers continuous and automated monitoring of UO, and it’s able to pick up on these changes “in real time and immediately alert the medical staff, while there is still time to intervene and prevent further damage.”
Sentinel offers a simple and cost-effective solution for the precise, continuous measurement of urine flow rate in real time.
“The device works in synergy with existing hospital equipment (any catheter and bag) and requires a short and simple, non-invasive installation,” the company said in a statement. “It fits the complicated ICU and operating room environments and is fully functional in any patient condition or hospital environment.”
Addressing when the device will hit the commercial market, Lark said that they hope to receive FDA clearance this year, which will be followed by initial sales in the US by the end of the year. The plan is to add additional countries pending regional regulatory approvals.
Asked what 2020 has in store for Serenno Medical, Lark told the Post that in addition to securing funding and strategic partnerships, the med-tech company “will focus on manufacturing of the Sentinel device for market penetration later this year.
“To support this process, we will also continue with additional clinical trials in Europe and the US,” he said.
Lark said that in 2021, we plan to add more features to the device, including the sampling of additional urine parameters and monitoring of Intra-Abdominal Pressure.
Founded in 2017, Serenno Medical is a portfolio company of Alon Medtech Ventures, owned by Dr. Shimon Eckhouse, a renowned entrepreneur and investor in the field of medical devices and medical technologies.
Tags
Who is online
42 visitors

Of course there will still be some who say they get no benefits from supporting Israel.
Israel's contributions to society have been significant, very significant!!!
List of Israeli inventions and discoveries